In this interview, Mateusz Filipiak, Scientist at the Cell and Gene Therapy Catapult (CGT Catapult), highlights one of our research projects involving the development of a smart bioprocessing platform for autologous cell therapy development.
1. Please provide an overview of the project presented in this poster
This poster highlights a project we are working on at the CGT Catapult as part of the SMARTER Consortium. This multi-partner collaboration is focused on developing a smart manufacturing platform for personalised cell therapies which leverages process analytical technologies (PATs) and closed-loop control systems for enhanced process monitoring and control in real time.
The aim is to improve manufacturing efficiency for autologous therapies, accounting for patient-patient variability, whilst also improving the quality of the final drug product.
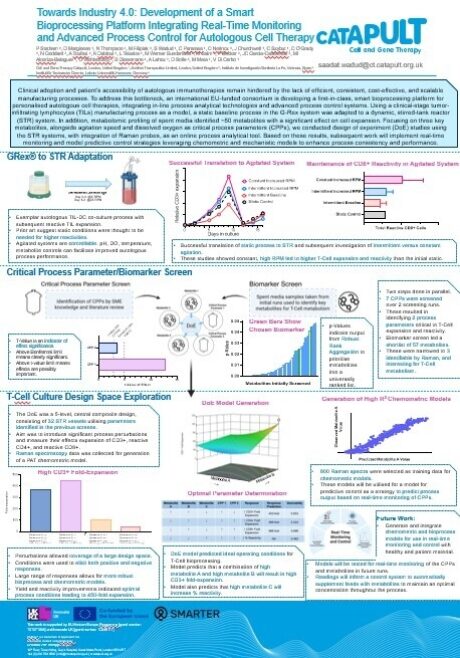
This poster highlights an aspect of this project, where we scaled a healthy donor model of a clinical-stage tumour-infiltrating lymphocytes (TILs) manufacturing process into a stirred tank reactor (STR). Key biomarkers and critical process parameters (CPPs) were identified, and later assessed in a design of experiments (DoE) study using an inline Raman sensor. The data generated has helped us to build chemometric and mechanistic models, improve process understanding, and enabled implementation of real-time in-process control strategies.
2. Please describe your role and how you were involved in this project
As a Scientist at the Cell and Gene Therapy Catapult, my focus areas include analytics and upstream bioprocessing.
As part of the SMARTER project, my role, alongside others in the team, was initially to scale up and optimise the TIL and dendritic cell (DC) process from a static system into an STR. This task required a high level of collaboration and attention to detail to ensure the cells maintained their reactivity against target antigens.
As the project progressed we then carried out a five-level central composite DoE across several process runs, assessing a range of CPPs informed by the literature, and expertise from our in-house team and consortium partners.
Learn more about the SMARTER Consortium
The project has been very hands-on, both in and outside the laboratory, involving the expansion and maintenance of the co-culture, frequent sampling, and analysis of the culture to ultimately generate meaningful data for the development of chemometric and mechanistic models.
3. What did you find most rewarding about working on this project?
It is rewarding to feel like you are working on something still in the early stages of development across the industry, where any discoveries or optimisations can be at the forefront of the field – especially when it comes to a process as complex as this!
Trying to balance high cellular fold expansion while retaining reactivity against antigens of interest is a significant challenge. Achieving this, where the resulting outcomes are improved therapies for patients, is very fulfilling.
It has also been rewarding to collaborate with world-class experts – people we might not otherwise get the opportunity to work with – and to exchange ideas on how we can tackle industry challenges involving smart bioprocessing platforms.
4. What challenges did you experience and how did you overcome these?
The baseline manufacturing process in the original static system was quite complex and scaling it up to an STR system presented an equally challenging task. Particularly in determining how to translate a static process into one requiring agitation without negatively impacting the cells or their reactivity. Fortunately, we were able to rely on deep expertise within the team to develop solutions and test various conditions to identify the most optimal approach.
The same level of complexity applied to planning and executing the DoE, which involved multiple testing levels to ensure robust conclusions. This resulted in a high number of experimental runs, each one needing to be completed without deviations. This challenge was successfully addressed by a well-trained team capable of handling these intricate runs, ensuring the process was as smooth and successful as possible.

5. How do you plan to build on the success of this project in the future?
The outcomes of this work lay a strong foundation for future projects focused on automated process control, a critical step toward making advanced therapies more scalable and accessible.
By gaining a deeper understanding of the key variables that influence cell culture performance, we’re better equipped to support the development of smarter, more responsive bioprocessing platforms. This not only opens up new collaboration opportunities for myself and the CGT Catapult, but also enables us to bring meaningful value to the industry – helping reduce production timelines, lower costs, and ultimately improve the consistency and quality of therapies delivered to patients.
Learn more about how we facilitate consortia