In this interview, Maria Barreira, Lead Scientist, highlights one of our Challenge Theme research projects, involving the development of a HEK293 cell line to support the production of more affordable and effective gene therapies.
1. What are the current challenges in achieving cost effective manufacturing of recombinant adeno-associated virus (rAAV) and why is it important that existing production methods are improved?
There are several key challenges to achieving cost-effective rAAV manufacturing: limited productivity, low global purification yields and reaching the stringent purity requirements necessary to meet therapeutic dose demands.
Overcoming these productivity challenges and establishing more effective production methods, which enable the sector to utilise approaches such as cell engineering and novel techniques for genetic material introduction, are critical to ensure broader access to rAAV-based cell therapies.
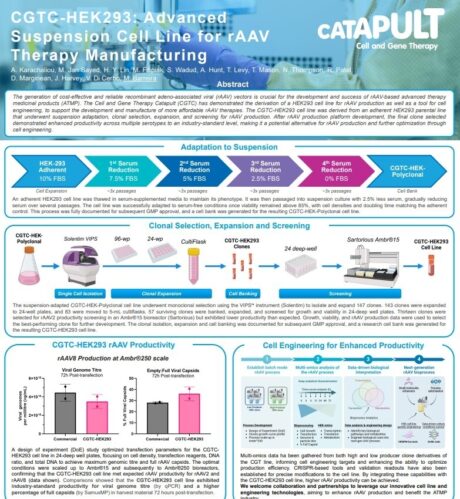
2. Please provide an overview of the project presented in this poster
This project focused on developing an accessible GMP-ready human embryonic kidney (HEK293) cell line and triple plasmid system, using innovative approaches which would enable game-changing productivity improvements in rAAV-based therapy manufacture.
To achieve this, we started by adapting an adherent HEK293 parental line to suspension growth, followed by clonal selection, expansion, and screening for rAAV production in 2D and 3D conditions.
The outcome of this project has been the development of a CGTC-HEK293 cell line which demonstrates industry-standard levels of productivity, providing a valuable alternative for rAAV manufacturing with unique opportunities for further optimisation.
3. Why have the CGT Catapult developed their own suspension HEK293 cell line for rAAV production?
Through the development of this cell line, we aim to improve the accessibility, affordability, and cost-effectiveness of rAAV production. This accessible cell platform enables the whole sector, from academics to gene therapy developers to CDMOs, to modify their rAAV development processes, offering unique cell engineering and deep characterisation capabilities to optimise manufacturing productivity and help meet the increasing demand for high-dose gene therapies.
Alongside the extensive opportunities for process optimisation, the use of this cell line also offers clear pathways for commercialisation and adoption, supported by a comprehensive repository of records to support the creation of future GMP cell banks.
4. What were the key challenges associated with developing this optimised HEK293 cell line, and how did you overcome these?
The first challenge we faced was transitioning the adherent HEK293 parental cells to a suspension culture while maintaining cell viability and growth consistency. This was addressed through a carefully controlled adaptation process, ensuring the cells retained their growth and productivity characteristics.
Clonal isolation and expansion posed additional challenges, requiring the development and testing of different strategies. Using specialised equipment such as Advanced Instruments’® VIPS® PRO, which enables precise single-cell deposition and high-resolution imaging, we were able to reliably identify high-performing clones with optimal productivity.
Enhancing rAAV productivity was another critical aspect, which was tackled using Design of Experiments (DoE) approaches and comprehensive screening methods using Sartorius’® Ambr®15 system. This technology enabled efficient, high-throughput parallel experimentation to optimise production parameters. Process scalability was subsequently demonstrated using Sartorius’® Ambr®250 system which facilitated the translation of optimised conditions from small-scale experiments to a scalable high throughput bioreactor process.
These approaches allowed for precise optimisation and scaling of production parameters, maximising cell line performance.
Learn more about our ATMP process technology capabilities
5. Improving the efficiency of AAV production is one of our Challenge Themes. How has the successful development of this cell line helped to address this challenge?
The development of this cell line enables us to test and apply advanced cell engineering techniques to further enhance rAAV yields and overcome productivity limitations. Additionally, making the cell line available to therapy developers and CDMOs reduces barriers to entry and facilitates more cost-effective rAAV manufacturing.
This advancement supports the broader gene therapy industry, accelerating the development of high-dose and high-demand therapies which can be accessed by more patients.
6. How can collaborators access and benefit from this capability?
Collaborators can access and evaluate our research cell bank for testing, along with optimised bench-scale protocols for shaker flasks and small-scale bioreactors. Interested partners, including therapy developers, CDMOs, and academic institutions, can engage with us to explore the potential of the cell line.
Furthermore, collaborators can work directly with us to broaden the cell line’s potential for engineering to achieve increased rAAV yields or stable expression. Collaborators could also benefit by assessing the cell line for production of therapeutic candidates and alternative AAV serotypes in the manufacturing industry.
Contact us if you are interested in this capability and would like to learn more
7. How do you plan to use this cell line and the learnings from this project to continue improving rAAV vector production across the gene therapy sector?
The insights gained from this project pave the way for identifying new opportunities in cell line engineering to boost rAAV production. By collecting multi-omics and process data from high and low producer clone derivatives of our HEK293 cell line, our team has created a dataset that integrates biological insights to optimise rAAV production processes and guide cell engineering targets. To further refine these efforts, we have developed CRISPR-based tools and analytics for validation.
We have also begun to incorporate this cell line into our next generation semi-continuous rAAV manufacturing platform, alongside our triple plasmid transfection system, to enable cost-effective manufacture of systemic and high dose rAAV-based treatments.
We now have the capability to build an extensive, high-quality dataset to establish digital twins for advanced rAAV manufacturing process control. Several collaborative projects in this space are already underway at our Process Analytical Technologies (PAT) Laboratory based in Stevenage.
One of these projects is focusing on the integration of automated and digital PATs within the rAAV manufacturing process, whilst a second project is working to enhance the digitised bioprocess sampling system in our PAT lab which can be used to explore the benefits of hybrid modelling for advanced therapy manufacturing.